https://en.wikipedia.org/wiki/Hyperoxia
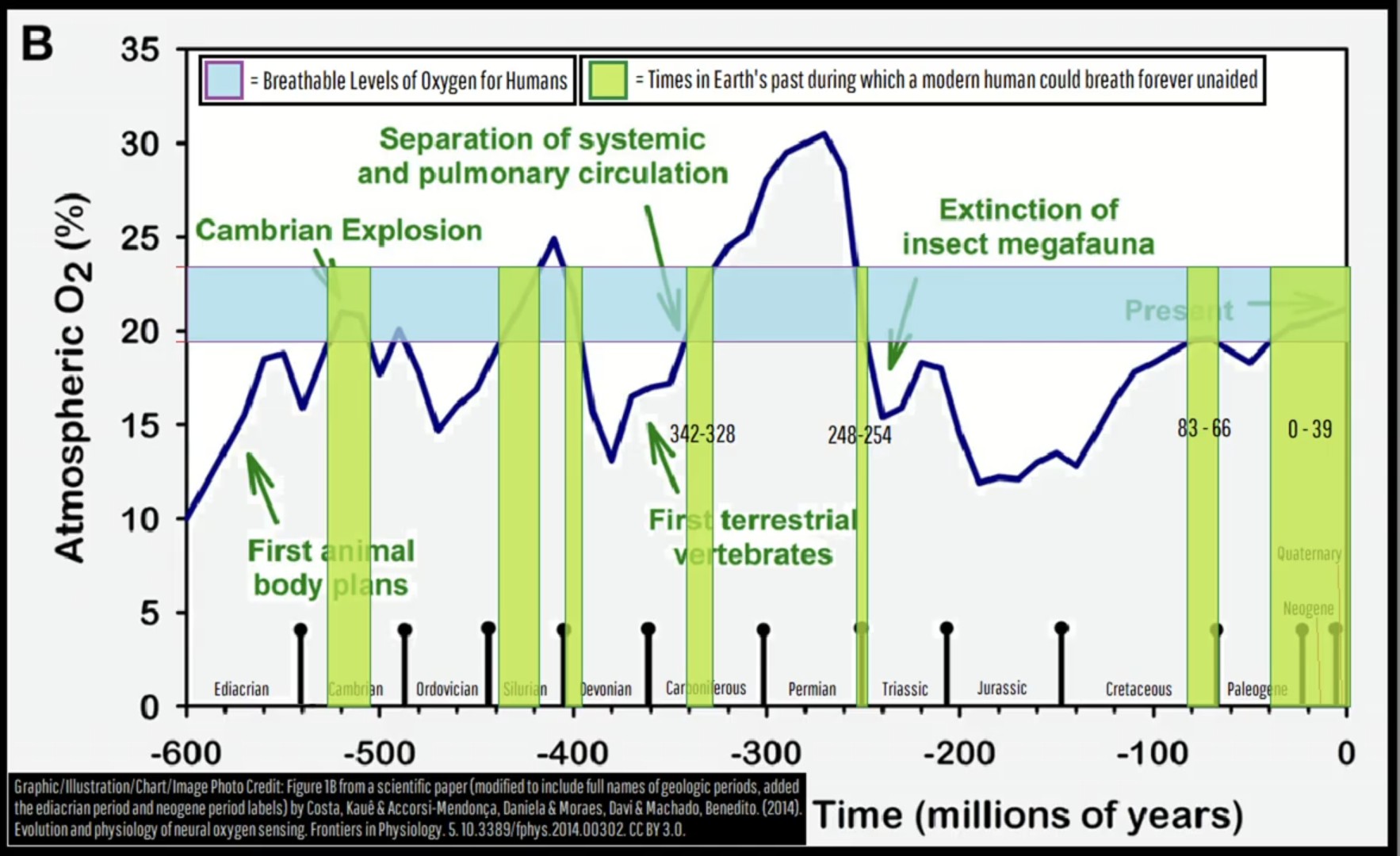
This was a screenshot I took months ago while watching a Geology Hub upload on YT. It was a lightbulb moment for my understanding of mass extinction events, (the largest was 250ma). I’ve referenced this multiple times, so thought I might share. Perhaps you find it as interesting as I do.
You can absolutely breathe higher partial oxygen pressure unaided for a long time. Hyperoxia isn’t all that lethal and definitely not quickly, if you’re only visiting, there’s no problem.
And if you want to live there, you should be much more worried about all the brand new diseases you don’t have immunity to, or the bugs that are bigger than you.
The low oxygen is definitely a problem, especially if you need to run away from the stuff mentioned above.
Also someone like a proper Sherpa sherpa from the Himalayas can function with oxygen that’s comparable to 7% sea level oxygen.
And there are towns with elevation so high the oxygen is equivalent to 15% sea level oxygen.
So this chart has pretty narrow limits. Sure, the legend does specify “breathe forever unaided”, but someone like a well accustomed sherpa who regularly climbs Mt Everest would have a much wider range than 20-25%
There are 850k people living in La Paz, Bolivia with the equivalent of 13.2% sea level oxygen and they seem to be doing just fine.
No, no. I’ve seen the chart. They’re definitely dead by now.
kagis
So, the answer is yes. The term “partial pressure” is often heard in this topic: as long as at least 0.2 bar of pressure are produced by oxygen alone, then you can add as much (or as little) as you want of a diluent gas on top of it. Of course this diluent gas has to be inert enough to avoid interfering with respiration, e.g. helium or nitrogen.
I don’t think that that’s authoritative as a lower limit – the guy is just saying that what matters is the partial pressure of oxygen. Assuming that that is right…
There is an upper limit on the partial pressure of oxygen. Above 1.6 bars oxygen toxicity becomes a problem.
Yup an a scuba diver, 1.6 at rest is the most we use while decompressing. 1.4 is the recommendation while exercising and 1.2 if you’re doing a long dive.
The military, as I understand it can push up to 2.0, but they have people that are super fit & they test people for oxygen tolerance too.
These aren’t gonna be hard limits, though, just maximums on what you’d subject a person to intentionally.
https://www.checkyourmath.com/convert/pressure/atmospheres_bars.php
There are 1.01325 bars in an atmosphere.
https://en.wikipedia.org/wiki/Death_zone
The concentration of oxygen (O2) in air is 20.9% so the partial pressure of O2 (PO2) at sea level is about 21.2 kPa (6.3 inHg; 3.07 psi).
Humans have survived for 2 years at 5,950 m (19,520 ft) [475 millibars (14.0 inHg; 6.89 psi) of atmospheric pressure], which appears to be near the limit of the permanently tolerable highest altitude.[13]
So “normal” at sea level is 1 atmosphere, with 20.9% of that being oxygen.
If WP is correct, we can get down to 46% of that partial pressure of oxygen at normal atmospheric mix at sea level (though we couldn’t be climbing mountains then, would cut into our survivable altitude range). So we could get down to 9.614% atmospheric oxygen and be okay at sea level.
https://en.wikipedia.org/wiki/Oxygen_toxicity
Lambertsen concluded in 1987 that 0.5 bar (50 kPa) could be tolerated indefinitely.
So that’d be 50.7% oxygen, or about 2.42 times the partial pressure of oxygen at sea level.
Going off that range – 9.614% to 50.7% oxygen at sea level – we could handle all of the oxygen levels on the chart. But two important caveats:
-
That’s only at sea level. The “dead zone” altitude on mountains and such would drop when the oxygen level is lower than it is in the current atmosphere.
-
We probably wouldn’t perform as well as we do today towards the extremes. It might be survivable, but “survivable” can be a long way from “biologically optimal”.
We probably wouldn’t perform as well as we do today towards the extremes. It might be survivable, but “survivable” can be a long way from “biologically optimal”.
I often wonder about this. Is the increase in CO2 making us dumber?
I’m certain the micro plastics and forever chemicals are messing with us majorly. It’s causing more than just higher cancer rates, but all these things are hard to tease out, especially since global we’re all being exposed to so many new poisons over the same time periods. It’s in the rain, the soil, the oceans, and everywhere else
It all just makes me think - how much potential are we each losing to subtle effects of pollution?
I’d think that the aerosolized lead from leaded gasoline did far worse than any plastic or change in CO₂. There’s still lots of volatile pollutants, like CO and hexavalent-anything out there.
-
or the bugs that are bigger than you.
Yea this was my takeaway. I think I’ll only travel to times after insect megafauna go extinct.
you should be much more worried about all the brand new diseases you don’t have immunity to
The diseases of amphibians, who are likely similiar to something you inherited from your mother already?
If my immunity works on diseases from the early Devonian period, I’m just gonna skip my next covid booster
We are merely the space ships of the microbes. Being caught between their war of the worlds would likely be unpleasant. However, I have a feeling we would be the true harbinger of death in that world of far more simple food webs than our present reality.
Our deaths would be miserable upon return, due to the evolutionary life of our passengers having many millions of years to plot our deaths when we return to find a completely different world and the little ones waiting to say hello.
I do not believe the table is intended for individual timescales, like how I tried to make the title more relatable. It is intended on geological timescales of species survival.
IIRC the person on YT also mentioned there were times when there was not enough oxygen to sustain fire, which is another fascinating thought and helps make sense of things like hydrocarbon deposits like oil and coal in some instances.
There are 850k people living in La Paz, Bolivia (elevation 3650m) with the equivalent of 13.2% sea level oxygen and they seem to be doing just fine. And granted, the natives of the region display some hemoglobin adaptation, but still… Even Aspen, Colorado sits at about 15% sea level oxygen and I’m pretty sure people don’t wear breathing gear while skiing there.
Altitude reduced the density of air as whole. The graphic is about changes in composition while density should remain similar, meaning if oxygen is low, something else (e.g. carbon mono-/dioxide) is high. So I wonder how this compares.
Partial pressure of the gases you breathe is what matters though, that’s why astronauts could breathe pure oxygen for days during the Mercury/Gemini/Apollo missions and be fine (as long as there’s no fire :/ ).
Partial pressure of the gases you breathe is what matters though
Just to clarify, the partial pressure of oxygen in the atmosphere is about 3 PSI. The partial pressure of Nitrogen in the atmosphere is about 10 PSI.
that’s why astronauts could breathe pure oxygen for days during the Mercury/Gemini/Apollo missions and be fine (as long as there’s no fire :/ ).
Fire risk is also related to the partial pressure of the gas. 100% oxygen is not actually a problem, so long as the partial pressure of that oxygen is low. The partial pressure of oxygen in the Apollo 1 capsule was about 16.7 PSI.
Astronauts still use 100% oxygen in space suits pressurized to about 5PSI.
The partial pressure of oxygen in the Apollo 1 capsule was about 16.7 PSI.
I had always assumed they just compensated with oxygen pressure to match atmospheric partial oxygen pressure, but I checked and indeed you’re right, but it was only that high an oxgen pressure for testing purposes on the ground. Once in flight, they would have dropped it to 5 PSI. It also makes sense as to why that Apollo 1 fire was so violent. Thanks for that learning opportunity ! :)
I was actually more referring to the chart implying that low oxygen means higher concentration of toxic gases
Though you start to notice it around 2500m, as a untrained person.
even earth feels like an alien planet millions of years ago, imagine if advanced space faring civilization do probe earth and categorized it to be inhospitable before, during and after their civilization crumbled to dust.
That also makes me wonder how we do our studies on other planets and if there is a category for “not habitable, but it could be”
Or similarly, “not habitable, but it could have been”. Subtle difference between the two.
We can just barely sometimes tell the difference between a small neptune, a large earth, an ocean planets, a coteless ocean planet, and a hycean planet. Trying to guage not just habitability but also terraformability is a ways off yet.
We can start with size and surface temperature though. We’re not going to survive on a gas giant, and anything with a four digit temperature is right out.
The atmospheric pressure needs to be significantly higher for even pure oxygen to put you at risk of oxygen toxicity. I don’t think this graph is accurate.
I think the white paper it came from is referenced in the bottom left box.
deleted by creator

Mega insects?!!!
Oh yeah, there was a whole era where the earth was extra oxygenated so you had XXL insects flying around. They have even done experiments raising modern insects in oxygen rich tanks and they grow (proportionally) quite large!
Yep.
DragonGryphonflys that had around a foot / 30 cm wingspan, and underwater scorpions that were 18 feet / 6 meters long including the tail.Okay, yeah, thanks for that nightmare …
Well to be fair squid get much bigger than 6 metres
True. But for the most part, squid are also not chittering outside at night, banging on the windows trying to get in.
Thank you. This will come in handy.
Thanks! I’ve always wondered about this. From the comments though, it sounds like there’s a few caveats with what “breathable” really means. Very very interesting.
i just wanna go back a couple decades to prevent a few of my mistakes, invest heavily in bitcoin and watch a couple dead singers perform
I read “space farting civilization”. That is an interesting concept.
That would make gravity just wafting farts.
At least we’d know that dark energy comes from some kind of hole.
This seems like something that should be printed on the back of business cards, or bus time tables.
You know, just in case.
Only for the oil industry business cards.
I’m just stuck at how to calculate the Earth’s rotation with the current cosmic expansion; turns out, combining galactic rotations and universal expansions isn’t as simple as you’d think! After that I’m off towards time traveling.
Problem with time travel is all the miscalculations trying to hit the thin skin of a moving object. Don’t forget the undulations through the galactic arms and the effect of the galactic medium against the heliopause. Good luck! Never know, maybe you’ll be the first above ground and non orbital. Bring a parachute, ablation shield, a shovel, and say hello to Hawking!
P.S. If you get a chance, tell me to call in sick for work 2/26/14. I’d appreciate not being disabled in that alt timeline. Thx!
A safer solution would be to just purposely target your arrival to the other side in empty space. While also travelling in a fully functional spaceship. Just make sure your little ship arrives within a set distance to Earth otherwise you will have to figure out how to travel millions of kilometers to get to where you’re going. If technology gets to the point of developing safe time travelling, I’ll assume that the same level of technology should be able to develop a space ship.
Even easier, send fast-return probes to collect a hard record of locations. Who cares if your prediction models are to chaotic? We’ve already seen the destination!